
(1/2) Battery lifespan, battery safety, and how high temperatures…
Microgrids with high renewable penetrations bring many benefits (such as reductions in costs and increased sustainability), however, they also come with many challenges. In particular, the integration of intermittent renewables (such as solar PV) requires the usage of energy storage. This is mainly done by utilizing batteries.
However, the conditions at many microgrid locations make energy storage challenging. At many locations where we work (such as Africa and the Middle East) the temperatures can be extremely high.
These high temperatures decrease the lifetime of most batteries dramatically. Both lead-acid and most lithium-ion batteries (the two most widely used battery chemistries) are very sensitive to this problem, as it is shown on [Fig. 1]. As battery banks are usually significantly costly, a shortening in the lifetime of this component may threaten the economic feasibility of a whole microgrid project.
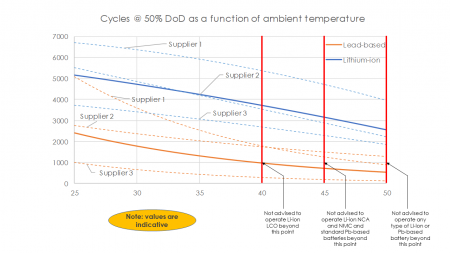
A few conclusions may be extracted from the chart [Fig. 1] shown:
- On average, lithium-ion batteries lifespan degrade
~30% if the room temperature is increased from 25°C to 40°C, and ~50% if the
room temperature is increased 25°C to 50°C.
- The best performing (in terms of high temperature performance) lithium-ion batteries degrade ~20% at 40°C and ~40% at 50°C.
- The worst performing (in terms of high temperature performance) lithium-ion batteries degrade ~35% at 40°C and ~60% at 50°C.
- Lead-acid batteries, which already have a lower
lifespan at 25°C (on average ~55% lower, even though it can be up to 80% lower
for certain models), have a worse behavior under high temperatures. On average,
lead-acid batteries lifespan degrade ~60% if the room temperature is increased
from 25°C to 40°C, and ~80% if the room temperature is increased 25°C to 50°C.
- The best performing (in terms of high temperature performance) lead-acid batteries degrade ~35% at 40°C and ~55% at 50°C. In general, this values correspond to high-temperature suited lead-acid batteries.
- The worst performing (in terms of high temperature performance) lead-acid batteries degrade ~70% at 40°C and ~85% at 50°C.
Another issue associated to battery energy storage at high temperatures is safety.
- Lithium-ion batteries present a risk of thermal
runaway, which increases with the temperature. A thermal runaway may provoke a
fire or even explosions. Within the lithium – ion chemistries:
- The LCO chemistry (lithium-cobalt-oxide) present the highest risk.
- The NCA (lithium-nickel-cobalt-aluminium-oxide) and NMC (lithium-nickel-manganese-cobalt-oxide) chemistries are relatively safe.
- The LFP (lithium-iron-phosphate) and LTO (lithium-titanate) chemistries are the safest within the lithium-ion technologies.
- Lead-acid batteries are thermally stable, thus, there is no risk of thermal runaway. However, if there is overcharging and overheating, the amount of H2 produced rises, creating a high risk of explosion if the space is not ventilated adequately. Furthermore, hydrogen sulfide (H2S), which is flammable and very poisonous, may be produced.
The good news is that there are solutions to overcome these problems and to be able to operate safely and efficiently at high temperatures, such as using alternative chemistries that are well-suited for high temperatures and/or using solutions to cool down the battery bank. In our next article about high-temperature batteries, we will discuss about these solutions in greater detail.
Disclaimer: the whole content of this article is for general information purposes. Gommyr Power Networks makes no representations or warranties of any kind, express or implied about the completeness, accuracy, reliability, suitability or availability of this information. Any reliance you place on such material for making any business, legal or any other decision is therefore strictly at your own risk.